Introduction to Brineura
Brineura (cerliponase alfa) is a significant medical advancement in the treatment of Late-infantile Neuronal Ceroid Lipofuscinosis Type 2 (CLN2), which is a rare neurodegenerative disorder commonly known as Batten disease. This condition leads to rapid neurological decline and affects children’s ability to see, move, and think clearly. The importance of Brineura cannot be understated, as it provides a life-altering option for patients, enhancing their quality of life and offering hope where few treatments previously existed.
What is Brineura?
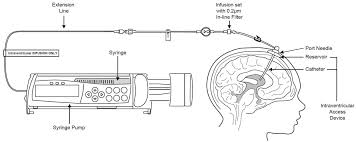
Brineura is an enzyme replacement therapy that works by providing a synthetic version of the enzyme that is deficient in CLN2 patients. Approved by Health Canada in 2017, Brineura is administered by intraventricular infusion directly into the cerebrospinal fluid, allowing the drug to bypass blood-brain barrier challenges and reach brain tissues more effectively. Clinical trials have demonstrated that Brineura can improve cognitive and motor functions, which is crucial for managing the debilitating effects of Batten disease.
Recent Developments and Updates
Since its approval, there have been significant developments in the application of Brineura. Researchers and healthcare providers have witnessed positive outcomes, with many patients showing stabilized or improved neurological function over time. Data from ongoing studies indicates that early administration of the drug yields better results, leading to ongoing efforts to raise awareness about the importance of early diagnosis. Collaborative initiatives among healthcare professionals are being promoted to ensure that patients receive timely treatment.
Challenges and Considerations
Despite its promise, Brineura treatment isn’t without challenges. There are logistical hurdles associated with the infusion process, including the need for specialized care teams and facilities to administer the therapy. Additionally, long-term effects and storage conditions for the drug necessitate careful management. Families have reported the emotional and financial burden related to treatment costs, which can be substantial, leading to calls for government and health insurance support to lessen the financial strain on families affected by CLN2.
Conclusion: A New Era for Batten Disease Treatment
Brineura represents a beacon of hope in the field of rare disease treatment. As research continues to unveil the full potential of enzyme replacement therapies, the significance of Brineura extends beyond its immediate benefits to patients. It catalyzes a movement towards increased research funding, greater disease awareness, and the potential for new therapies that could help countless children battling neurodegenerative diseases. For families affected by CLN2, Brineura is a ray of hope in an otherwise challenging landscape.